Recent literature has shown that loss-of-function of BRD9, a component of the noncanonical SWI/SNF (ncSWI/SNF) complex, mediated either by RNA interference or by BRD9 tool degrader compounds can inhibit multiple myeloma (MM) cell lines and primary MM cell proliferation in vitro, as well as inhibit mouse MM xenograft tumor growth in vivo. Additionally, in vitro synergy in MM proliferation assays was observed when a tool BRD9 degrader was combined with the standard of care (SoC) agents, dexamethasone or pomalidomide. Given the continued unmet need of the MM patient population, we explored the therapeutic potential of CFT8634 in this indication. CFT8634, a potent and selective oral BiDAC™ degrader of BRD9, is currently in a Phase 1/2 clinical trial (NCT05355753) for the treatment of SMARCB1-perturbed cancers, including synovial sarcoma and SMARCB1-null tumors. Pharmacokinetic and pharmacodynamic data to date from the ongoing trial of CFT8634 has demonstrated dose-proportional human plasma exposure and robust BRD9 degradation in patient tumor samples thereby establishing proof of mechanism in patients.
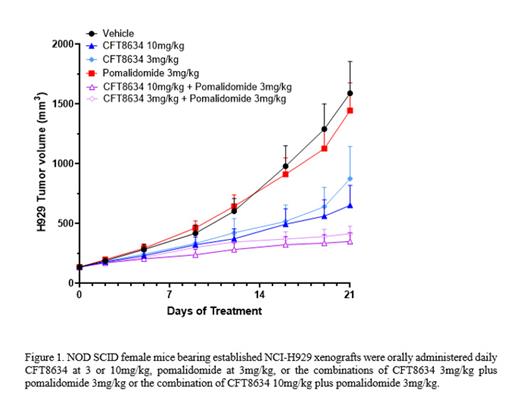
Here we show that CFT8634 has anti-proliferative activity in a subset of MM cell lines that translates into tumor growth inhibition in MM xenograft models at clinically relevant CFT8634 exposures. We observe that cell lines less sensitive to pomalidomide tend to be significantly more sensitive to CFT8634 treatment. As pomalidomide is a SoC treatment in MM, we interrogated the ability to combine CFT8634 and pomalidomide. CFT8634 demonstrates synergy with pomalidomide in MM cell lines where CFT8634 is anti-proliferative as a single agent. We further show that clinically relevant exposures of CFT8634 demonstrate synergy with pomalidomide in vivo in the NCI-H929 mouse xenograft model (Figure 1). This model is relatively unresponsive to pomalidomide alone, suggesting that CFT8634 can render otherwise recalcitrant MM tumors sensitive to the effects of pomalidomide in combination. Pharmacokinetic and pharmacodynamic analyses show that in combination, CFT8634 and pomalidomide do not interfere with the ability to degrade each other's targets (BRD9 and IKZF1/3, respectively) despite both utilizing the E3 ligase cereblon.
In conclusion, we demonstrate in vitro and in vivo activity in an expanded set of MM models using the clinical oral BRD9 degrader CFT8634 as a single agent and show in vivo synergy between CFT8634 and pomalidomide at clinically relevant doses. Our findings suggest that combining CFT8634 with pomalidomide may be a viable therapeutic strategy in treating MM patients.
Disclosures
Poling:C4 Therapeutics: Current Employment, Current equity holder in publicly-traded company. Cocozziello:C4 Therapeutics: Current Employment, Current equity holder in publicly-traded company. He:C4 Therapeutics: Current Employment, Current equity holder in publicly-traded company. Hurh:C4 Therapeutics: Current Employment, Current equity holder in publicly-traded company. Lobbardi:C4 Therapeutics: Current Employment, Current equity holder in publicly-traded company; Blueprint Medicines: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Jackson:C4 Therapeutics: Current Employment, Current equity holder in publicly-traded company. Fisher:C4 Therapeutics: Current Employment, Current equity holder in publicly-traded company. Pollock:C4 Therapeutics: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal